×
News

24
2022
-
11
The originator of domestic cardiovascular intervention submitted the listing application, and the overseas revenue of Yeju Medical accounted for more than 80%
Yeju Medical Group Holdings, a leading vascular intervention company, filed an application with the Stock Exchange of Hong Kong on Nov 23 to list on the main board.
Author:
Yeju Medical Group Holdings, a leading vascular intervention company, filed an application with the Stock Exchange of Hong Kong on Nov 23 to list on the main board.
Data show that Yeju Medical is a global leader in the development and manufacturing of PCI/PTA balloon, mainly manufacturing percutaneous coronary intervention (PCI) and percutaneous intravascular angioplasty (PTA) intervention instruments, and its PCI/PTA balloon products have been widely used in more than 70 countries and regions around the world.
In terms of PCI balloon sales in 2021, Yeju Medical occupies a leading position in major global markets, such as the second place in Japan, the fourth place in Europe, and the sixth place in the United States and ChinaIs the only company headquartered in China, and can in Europe, the United States, Japan and other major overseas PCI market share in the top six enterprises.In the PTA balloon market, Yeju Medical also occupies a dominant position. Its product sales rank third in Japan and fourth in the United States.
As of June 30, 2022, Yeju Medical has more than 40 approved products, including 25 PMDA approved products in Japan, 22 CE Mark products in the European Union, 14 FDA approved products in the United States and 15 products approved by the National Food and Drug Administration.
Among them, Yeju medical products mainly include semi-compliant balloons, special catheters, notched balloons, non-compliant balloons, etc., covering all the major therapeutic processes in PCI and PTA surgeries, such as entering the lesion, preparing the lesion, treating the lesion, and optimizing the lesion.
In addition to the two segments of PCI/PTA balloon, Yeju Medical is also actively expanding its business in neurovascular intervention and structural heart disease. For example, Yeju Medical is developing innovative products such as valvuloplasty balloon catheter, balloon dilated heart valve, next generation heart valve, nerve balloon, nerve microcatheter, nerve plugging balloon and so on.
Based on its leading position in the global PCI/PTA device market, Yeju Medical currently has excellent financial data; Based on the forward-looking layout in the field of neurological intervention and structural heart disease, Yeju Medical is expected to usher in rapid revenue growth in the future.
PCI/PTA products are the two biggest cash cow, 2021 adjusted profit exploded 200% year on year
According to the prospectus, Yeju Medical will have revenue of $96.34 million in 2019, $88.47 million in 2020 and $116 million in 2021. In the first half of 2021 and 2022, its revenue was approximately $57.34 million and $68.85 million, respectively.
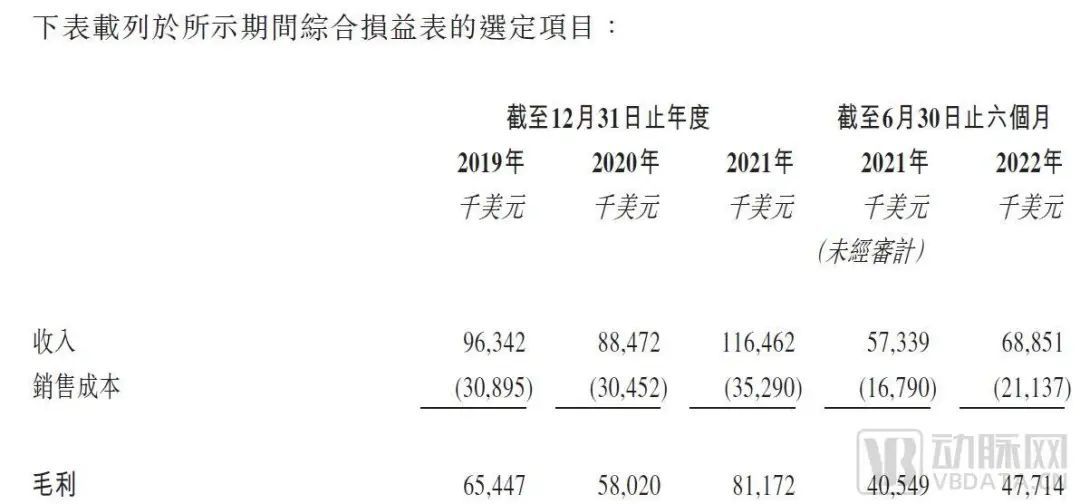
Yeju medical financial data
The decrease in the revenue of Yeju Medical in 2020 is due to the decrease in PCI operations worldwide due to the impact of the pandemic. However,As the epidemic stabilizes, Yeju Medical's revenue grew 31.6% in 2021 and 20.1% in the first half of 2022 compared to the same period.
In addition, compared with other unprofitable listed medical device enterprises, Yeju Medical has strong profitability. According to the prospectus,Its adjusted annual profit for 2021 is approximately $21.4 million, up 201.4% from 2020;In the first half of 2022, adjusted profit increased 23.6% year on year to $13.6 million on a high base.
The reason for achieving such outstanding financial performance is that Yeju Medical has three core competencies.
First, Yeju Medical has a strong ability of innovation and research and development, and the product advantages are obvious.
In research and development, Yeju Medical has created a variety of proprietary technologies, such as the world's leading antibody coating technology with the function of "promoting healing". Based on technological innovation, Yeju Medical has applied for more than 100 licensed patents in major jurisdictions around the world, including 32 in the United States and 45 in China.
In terms of product advantages, Yeju Medical has innovatively developed a number of world-first products. For example, its COMBO Plus double therapy stent is the world's first and only commercial "drug plus antibody" double-coated stent that effectively promotes coronary vascular healing. Scoreflex NC is a non-compliant notch balloon with the smallest external diameter. Sapphire II Pro is the first FDA-approved 1.0mm diameter balloon; JADE Non-compliant Peripheral Balloon is the first and only FDA-approved non-compliant integral Exchange PTA balloon compatible with all guide wire systems...
Based on multiple innovations, Yeju Medical's products have been widely recognized by clinical experts around the world. From the revenue data, the overseas revenue of Yeju Medical accounted for 80%-95% of the total revenue in the first half of 2019-2022. In the first half of 2022, Japan and the Europe, Middle East and Africa (EMEA) region accounted for about 25% and 24% of total revenue, respectively, while the US market, which it entered only in 2017, saw its share of revenue jump from 4.5% in 2019 to about 10.2% in the first half of 2022.It can be seen that the products and brands of Yeju Medical are well recognized in the developed markets.It can be seen that the products and brands of Yeju Medical are well recognized in the developed markets.
Second, Yeju Medical Therapy has a strong global commercialization ability.
Up to now, Yeju Medical has built a complete "direct sales + distribution" global sales network, so that the products can be sold to more than 70 countries and regions. Based on efficient management of the direct sales team and distribution team, the sales volume of PCI balloon products of Yeju Medical ranked second in Japan and fourth in Europe in 2021. PTA balloon product sales in 2021 ranked third in Japan and fourth in the United States.
It is worth mentioning that PCI and PTA balloon markets are highly concentrated. For example, four companies monopolized 88% of the PCI balloon market in Japan, and six companies monopolized 97% of the PCI balloon market in Europe. Five companies monopolize 97% of the PTA balloon market in Europe... Among them, can be in the forefront of the industry, with a number of medical device giants,reflect Yeju Medical has strong global capabilities.

Third, Yeju Medical has high standard mass production capacity.
On the one hand, the production plants of Yeju Medical in China and the Netherlands have a strict and perfect quality management system, and has passed the National Food and Drug Administration, the United States FDA, Japan PMDA, the European Union notice body and other regulatory agencies audit and inspection. For example, in late December 2020, its facility received a pre-PMA inspection from the FDA, and the result was NAI (no corrective action required).
On the other hand, the total annual production capacity of the plant is about 1.35 million pieces of balloon products and 56,000 pieces of stent products, which can provide a stable and high-quality product supply to global customers, while also leveraging the advantages of scale.
Based on the above three points, Yeju Medical currently occupies a dominant position in the global PCI/PTA balloon market, and has achieved substantial revenues and profits.In the long run, the global PCI and PTA balloon market will grow steadily, and the PCI and PTA balloon business of Yeju Medical has a large space for development.
Currently, there are about 330 million cardiovascular disease patients in China, and the prevalence rate continues to rise, and the demand for treatment is growing. Compared with traditional thoracotomy, PCI/PTA surgery is more and more favored by doctors and patients due to its advantages of minimally invasive, safe and effective. At the same time, with the gradual improvement of doctors' understanding of transcatheter surgery, more and more hospitals will adopt PCI and PTA surgery. Therefore, the PCI/PTA device market will continue to grow in the future.
According to the prospectus, the market for PCI intervention devices in China, the United States and Europe is expected to grow at a compound annual growth rate of 14.0%, 13.1% and 10.0%, respectively, in 2021-2025. The PTA interventional device market will grow at a compound annual growth rate of 14.6%, 11.9% and 9.2%, respectively.
With the steady growth of PCI/PTA interventional device market, Yeju Medical will improve market share and steadily increase revenue by virtue of its dominant market position and product leadership PCI interventional devices, PTA interventional devices two business lines will also incarnation of the two "cash cow", become the most stable foundation for the development and innovation of Yeju medical.
With structural heart disease and neurological intervention, Yeju Medical creates a new revenue growth engine
Compared with the strong global commercialization capability, the research and development strength of Yeju Medical is no less.
On the one hand, the R&D team of Yeju Medical, which has accumulated 20 years of development experience, is located in Florida, USA and Shenzhen, China. They are not only experienced, but also have an international vision. On the other hand, Yeju Medical attaches great importance to technological innovation and spends more than 10% of its total revenue on R&D every year. For example, from 2019 to 2021, the R&D investment of Yeju Medical accounted for 10.0 percent, 14.2 percent and 10.4 percent of the total revenue in the same period, respectively.
Up to now, Yeju Medical has about 40 products under development, and distributes PCI/PTA devices and other products synchronously through horizontal and vertical two-way innovation.
Lengthwise,Through the concept of "making complex into simple", Yeju Medical continues to expand different product lines and upgrade the existing product portfolio to build a diversified PCI/PTA surgical product portfolio.


Specifically, Yeju medical continuityUpgrade existing products,For example, it is developing the fourth generation Sapphire balloon series, the next generation Jade II series PTA balloon, Teleport II microcatheter and other products, and customized ScoreFlex II series notch balloon for the Japanese market.
The industry gathers and the medical treatment returnsExpand different product lines,
As it has developed an ECMO left ventricular assist device, which is currently in the preclinical stage; A new generation of rapamycin drug-eluting balloon products is being developed to accurately deliver sufficient doses of active pharmaceutical ingredients to the focal site to reduce the risk of thrombosis, promote focal repair, and improve surgical safety and effectiveness. In addition, Yeju Medical is developing a CTO toolbox that includes products such as microcatheters, microcatheters with controllable tip shapes, guided extension catheters, and double-cavity microcatheters to help simplify complex interventional procedures.
Horizontally, Yeju Medical uses its sales network and R&D advantages in the field of PCI/PTA devices to distribute structured cardiac intervention products and neurological intervention products. For example, doctors of structural heart disease and coronary interventional doctors are the same group, so Yeju Medical can tap the existing sales network potential, and the principle of products in the field of neural intervention is similar to peripheral intervention of coronary intervention. Certain similarities can perfectly transplant the technology and commercialization advantages of Yeju Medical to the field of structural heart disease and neural intervention.


In the area of interventional structural heart disease,
In 2020, Yeju Medical established a joint venture with partners to develop, manufacture and market heart valve products. The joint venture's first commercial product, TricValve, is the world's first pre-loaded upper and lower double vena cava valve implant for the treatment of severe tricuspid regurgitation. It also has a well-established pipeline product. Such as aortic valve, mitral valve replacement, pulmonary valve replacement products. In addition to the pipeline products being developed by the joint venture, Yeju Medical is also working with its partners to develop balloon dilated heart valves, next-generation heart valves and other products.
In the field of nerve intervention, Yeju Medical's nerve balloon, nerve microcatheter, nerve blocking balloon, nerve drug coating balloon and other products have entered different stages of research and development or registration, among which the nerve balloon is expected to be approved around the end of the year. At the same time, Yeju Medical is also developing a variety of innovative products such as nerve suction catheter, nerve infarction balloon catheter, nerve thrombectomy device and blood flow guiding device. Based on the platform layout, Yeju Medical will enter the neurological intervention market faster.
At present, structural cardiology intervention and neural intervention are in a period of rapid growth, and the market space is more than 10 billion dollars. Cauterization consulting reports show that the penetration rate of interventional surgery for structural heart disease is low and is entering an explosive period. The market size of interventional devices for structural heart disease in Asia Pacific, including Japan and China, is estimated at $1.1 billion in 2021 and is expected to reach about $13.45 billion in 2030, with a compound growth rate of 32% from 2021 to 2030.
According to a report by Jirsense Consulting, the global market for neurointerventional devices reached $2.64 billion in 2021 and is expected to reach $4.43 billion in 2025.
With the rapid expansion of market size, the industry will accelerate the growth of enterprises. Among them, Yeju Medical is expected to rapidly enter into the global structural cardiology interventional and neurological interventional markets with the clinical advantages of the next generation of products and strong global commercialization capabilities, and achieve substantial revenues.
Based on this, we believe that with the launch of innovative products in the field of structural cardiology intervention and neurological intervention, Yeju Medical will add two new powerful engines for rapid expansion of enterprise scale.
In general, at present, Yeju Medical has PCI/PTA interventional device business two "cash cow". In the future, structural cardiac intervention and neurological intervention business will become the new growth point of its revenue, and together with PCI/PTA interventional device business constitute the four cash pillars, promoting the stable and rapid development of Yeju Medical.
(Note: Adjusted profit refers to the deduction of non-recurring expenses, which are the impact of changes in the fair value of preferred stock, listing related expenses, share-based compensation expenses, and commodity-linked fixed interest rate notes in the normal listing process of the company, and do not affect the normal operation of the company.)
Source:
Related news

