×
News

22
2022
-
11
The CroiValve Duo Transcatheter Tricuspid valve Repair system was successfully implanted in humans for the first time
CroiValve has announced that its Duo tricuspid valve repair technology has been successfully implanted in humans for the first time to treat tricuspid regurgitation (TR) as part of its TANDEM I study in Poland.
Author:

The implant team at the National Institute of Cardiology in Warsaw is pictured with Adam Witkowski and Maciej D Ay browski
CroiValve has announced that its Duo tricuspid valve repair technology has been successfully implanted in humans for the first time to treat tricuspid regurgitation (TR) as part of its TANDEM I study in Poland.
The procedure was performed as part of an ongoing clinical trial by Adam Witkowski and Maciej D Education browski at the National Institute of Cardiology in Warsaw, Poland and the Medical University of Silesia in Katowice, Poland.
The Duo system includes a valve implant that works in conjunction with the native tricuspid valve to restore valve function. The device uses minimally invasive techniques and is secured to the superior vena cava by a novel anchoring system, leaving the fragile right ventricle and the native valve device unaffected. The system is simple to implant, uses standard imaging techniques and is suitable for a wide range of patients, including those who are difficult to treat with other valve repair and replacement techniques.
Early cases have shown that Duo system is very effective in treating TR and can significantly improve the symptoms of patients. "The Duo system is unique in that it provides a solution to effectively treat the dilated right heart anatomy associated with tricuspid regurgitation while avoiding contact with the right heart to maintain normal heart movement," Witkowski said. After 30 days, our patient had already experienced a shift in symptoms, highlighted by the Ksas Cardiomyopathy Questionnaire (KCCQ) Quality of Life Survey and the drop from NYHA Level III to NYHA Level II as well as significant improvement in the six-minute walking test. In addition, the device continues to demonstrate excellent efficacy. The early use of the Duo system has shown that it is an easy-to-use, safe and effective device, and although this is the first time we have used the Duo system, the procedure is very simple, as shown by the short operation time. This is a stepwise controlled approach using standard ultrasound imaging that allows us to treat patients we would otherwise have no choice in."
About CroiValve

CroiValve Inc. is offering a new transcatheter for the treatment of tricuspid regurgitation. CroiValve is a simple, safe and effective way.
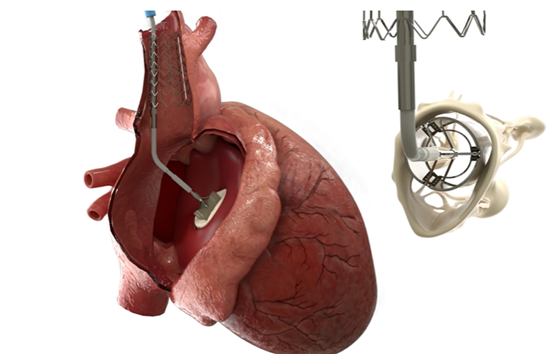

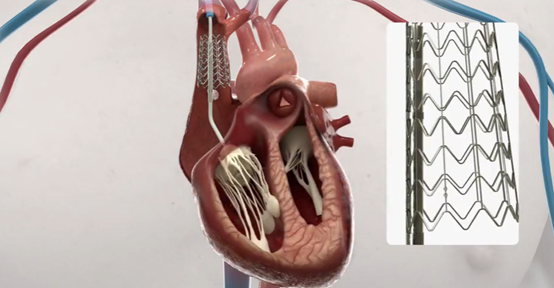
The CroiValve consists of a bracket, a connector, and a valve. The CroiValve system is designed to assist in dilated tricuspid valve engagement. The CroiValve is anchored within the tricuspid valve via a stent, while the valve is positioned between the primary lobes to reduce the size of the regurgitation hole and provide a contact surface for valve engagement. In addition to a simple space occupying design, the CroiValve has the internal structure of a three-lobed valve to support prediastolic blood flow through the tricuspid valve, thus reducing the risk of instrument clots. The new placement and fixation method of the CroiValve simplifies the procedure. The use of this new fixation method, rather than the traditional through-tissue anchoring method, makes the CroiValve safer and allows the CroiValve to be removed after surgery, which is very important (biological valves have a short life span and are not available to patients prior to the end of the year, which is a headache).
About CroiValve Corporation

CroiValveCroiValve is an early stage medical device company dedicated to developing minimally invasive devices for the treatment of tricuspid valve insufficiency. Based in Dublin, Ireland, the company combines extensive clinical, technical and commercial experience. Relying on technology from Trinity College's Center for Bioengineering, the Company is developing a minimally invasive device for the treatment of "tricuspid regurgitum," which can be delivered intravenically into the right ventricle. The device fills the space between the valve lobules, thereby preventing regurgitum. This program can treat the condition quickly and economically, without the need for a long hospital stay.
Source:
Related news

